Article contents
Quantitative assessment of degradation, cytocompatibility, and in vivo bone regeneration of silicon-incorporated magnesium phosphate bioceramics
Published online by Cambridge University Press: 30 December 2019
Abstract
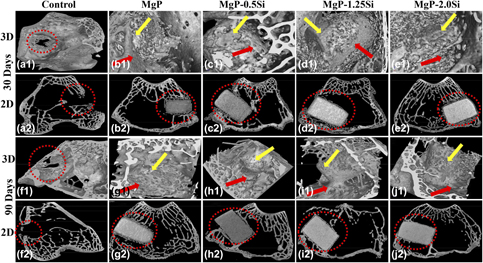
The effects of silicon incorporation on the in vitro and in vivo properties of magnesium phosphate (MgP) bioceramics were studied. Samples were prepared by conventional solid state synthesis method. Scanning electron microscopy and micro-computed tomography (µ-CT) analysis showed that Si doping reduces degradability of MgP. In vitro studies have shown that MG63 cells can attach and proliferate on MgP samples. Live/dead imaging showed that MgP–0.5Si sample had highest cell proliferation, which was also quantitatively confirmed by alamar blue assay. In vivo biocompatibility of MgP ceramics was assessed after implantation in rabbit model. Detailed µ-CT analysis showed new bone tissue formation around the implant after 30 and 90 days. MgP–0.5Si ceramics had 84% bone regeneration compared with 56% for pure MgP ceramics, as confirmed by oxytetracycline labeling. Our finding suggests that Si doping can alter physicochemical properties of MgP ceramics and promotes osseointegration, which can be a useful choice for bone tissue engineering.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
These authors contributed equally to this work.
References
- 10
- Cited by




